INTRODUCTION
The purpose of this experiment is to measure the difference in temperature (ΔT) between a cup of cooling water and the room as a function of time. You will then see if the data are consistent with Newton's law of cooling, which predicts ΔT to be an exponential function of the elapsed time.
THEORY
Newton suggested that the rate of heat flow from a hot body (that is, Q/Δt, where t represents time) is roughly proportional to the difference in temperature between the body's temperature (Twater, if that body is water) and the temperature of the surroundings, Troom. Since we often recognize heat flow by a temperature change (Q/Δt is related to T/Δt), Newton's law of cooling simply says that T/Δt = k(Twater - Troom), where k is a constant. Since Troom is constant, ΔT = (Twater - Troom) so we can write:
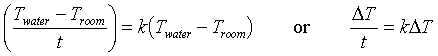
Thus the rate at which (Twater - Troom) changes is proportional to (Twater - Troom). When a quantity varies in this way it is said to vary exponentially with time:
or
where To is the initial change in temperature, t is the elapsed time, and τ is called the time constant. τ is the amount of time for the temperature to "decay" to 37% of the original To.
EXPERIMENT
Set up the following data table in your notebook. The elapsed time is the time that has passed since your first observation. Note that your first measurement occurs at t = 0!
|
Elapsed time, t (min) |
Twater (°C) |
Troom (°C) |
ΔT = Twater – Troom (°C) |
| 0.0 | — | — | — |
| 0.5 | — | — | — |
| … | — | — | — |
| 20.0 | — | — | — |
ANALYSIS
REPORT
|
|
||
| © | St. Lawrence University | Department of Physics |
| Revised: 25 Aug 21 | Canton, NY 13617 |