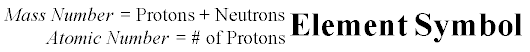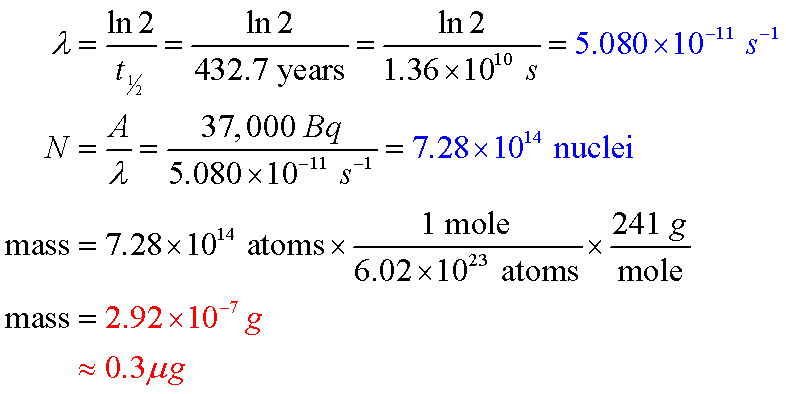- Computers are needed for lab this week
- The Lab Practical is in 2 weeks. Here's what you should tell them:
- There are only 3 experiments that they will perform individually during the practical this semester:
- Circuits: Given an unknown resistor, know how to connect it to a circuit and connect meters to read current and voltage; know how to use KaleidaGraph to analyze the data; know how to get resistance from a meter and the color codes (the color codes do not need to be memorized; just the procedure for getting resistance from the colors)
- Practice materials available: DC power supply, multimeters, 100kΩ resistor. Students can take wires needed from the rack in the lab
- Polarity of Unknown Magnet: Given a magnet with the ends obscured, determine the north and south ends. Remind them that they will be using the same galvanometer and coil as during the Lenz's Law experiment, but it may not be connected the same way, but that shouldn't matter as long as they remember the relationship between B, Bind and whether the flux increased or decreased
- Practice materials available: Galvanometer, red coil, red bar magnet. Take wires as needed
- Wavelength of Unknown Laser: Given a laser not used before and grating with known spacing, determine the wavelength of the laser
- Practice materials available: None available, but students should refer to the two experiments where they used this procedure: Double Slit Interference (determined λ of laser with known d) and Hydrogen Spectrum (determining d from known λ)
- Also, tell students they will NOT have to perform any error analysis other than what we've always done with the uncertainties and residuals in KaleidaGraph! Next week we'll perform the Lens Optics experiment, and they'll use KaleidaGraph during that lab
- Three copies of a No Eating/Drinking sign should be printed: two taped up outside the lab, one on the blackboard
- Logger Pro does not see the radiation monitor when the program is launched (the green 'Collect' button is grayed out) Students should first open the file from the T:\Phys152 drive, after which a 'Sensor Confirmation' window will pop up. Students click the Connect button, and all is well. This has been included in the lab instructions, which they are unlikely to have read
- We have six new Vernier Radiation Monitors with a mute switch, which should be turned off. The older versions lacked this switch and chirped incessantly.
- This is the first time that Phys152 uses Logger Pro, but some may have used it in the Gen. Chem. class
- Since I seem to have a permanent case of Covid-brain, here's the naming scheme for isotopes (this is also written on the blackboard):
- The latest version of the chart of the nuclides (Seventeenth Edition - Revised to 2009) is used. These charts are huge and nearly cover the entire bench:
 |
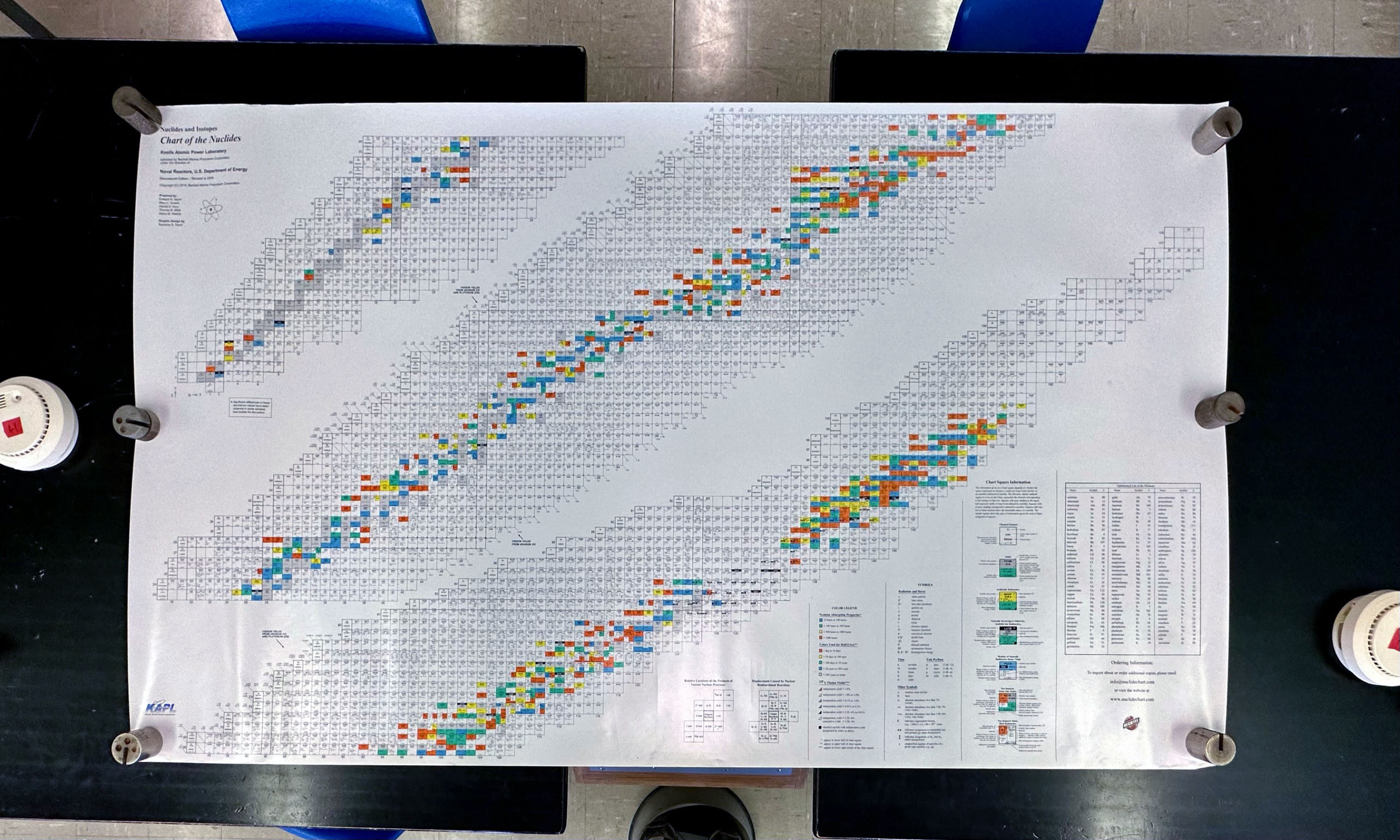 |
| The chart revision date is in the upper left corner |
Masses are used to hold the chart flat |
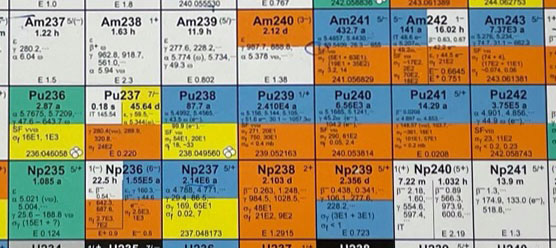
- Students find Americium on the chart of nuclides with atomic number 95:
- Students are looking for isotopes of Americium that are stable (long half-life, t½) and have a stable decay product. They should find from the chart of nuclides that Am 241 is the most likely substance to be in the smoke detector, since it has t½ = 432 years, and it decays to Np 237 (t½ = 2.4×106 years). After they have discovered the correct isotope, you can show them that "Am241" is printed on the metal cap of the ionization chamber
- Some will be able to figure out the nuclide chart for themselves, but many will need to be walked through it
- Using the board example (Am 239), ask students what the decay product is (Np 235). Point out that this is down two blocks, and over to the left two blocks, which will reveal the decay product due to alpha emission.
- Next ask which are the most likely Am isotopes; they should tell you Am 241, 242 & 243 since their half-lives are on the order of years (a on the chart), not days (d) or hours (h). Then have students look at their respective decay products (Np 237, 238, 239) to find out which one is most stable.
- There is a very large discrepancy between the published activity rate (37 kBq) and the measured rate with the detector directly on the source (~500-600 Bq). There are two reasons that might explain this: (1) there is still a small air gap between the source and detector; (2) the published rate is for radiation emitted in all directions around a spherical source, and the detector is just measuring a small region
- The mean number of counts will often be higher for 2 or 3 sheets of paper than 1 sheet. Remind students that they're only taking one minute of measurements. If they collected more data the results might be more like they expect
- If students bother to read the instructions, they will refer back to the nuclides chart to find out why the counts are not zero, even when 3 sheets of paper are placed between the source and monitor. Am 241 is also a weak gamma (γ) ray emitter. Feel free to insert your own "Hulk, Smash!" joke
- Here is a copy of my Logger Pro output, with calculations
- Weak students will think that λ means they will be calculating a wavelength. *sigh*. Gently remind them that λ is the decay constant
- In case anyone asks you about "The Radioactive Boyscout" (a teen who collected radioactive material to create a feeder reactor in a shed in the back of his mother's house), here's some pertinent reading:
- "David Hahn" - Wikipedia article about the person who collected radioactive material
- "The Radioactive Boy Scout" - The original article by Ken Silverstein that appeared in Harper's Magazine (November 1998)
- Using the published activity rate (also on the cap) of 37 kBq, they will calculate a mass of about 0.3 μg:
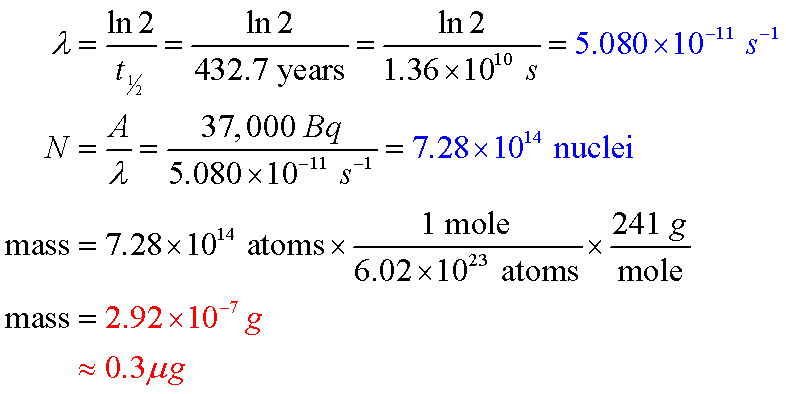
|